Proteins are formed from the amino acid residues and they have different structures and properties. They are very important in the body they function as enzymes, are used for support and sometimes for energy, and used as chemical messengers such as hormones. These are just some on the uses of proteins they are of much importance in biochemistry. The structures of proteins can be classified as follows:
i)Primary structure: usually referred to as the (1ο structure), it is the amino acid sequence of the polypeptide chains in a protein
ii)Secondary structure: usually referred to as the (2o structure), there are two structures that are commonly seen these are the alpha (α) helix and beta (β) sheet. They are usually associated with the hydrogen bonds between the amino acids, the amino acids are placed in an order with respect to their relative side chains.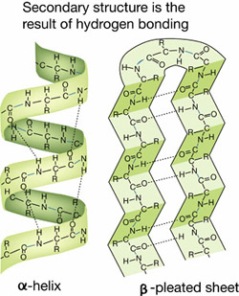
iii)Tertiary structure: usually referred to as (3o structure), this is the (3D) three dimensional structure of polypeptide chain.
iv) Quaternary structure: usually referred to as (4o structure), proteins are made up of more than 1 polypeptide chain which are called subunits. These sub units are associated by covalent bonds called di sulfide bonds, (formed between 2 sulphur atoms) . The 4ostructure of a protein is the layout of its subunits. 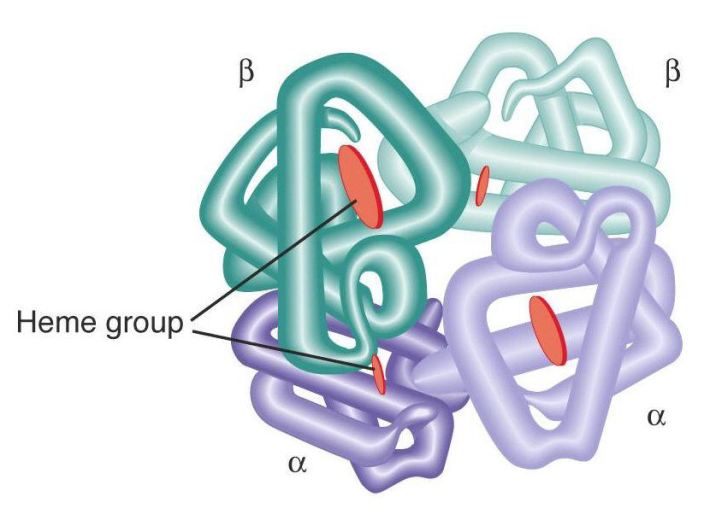
Proteins have many properties they were of much attention and were thought to be the genetic material before the discovery of DNA . They are sequenced by using X-ray crystallography which helps determine the amino acid groups that they are composed of. Proteins like other biological molecules can be broken down or denatured by certain factors.
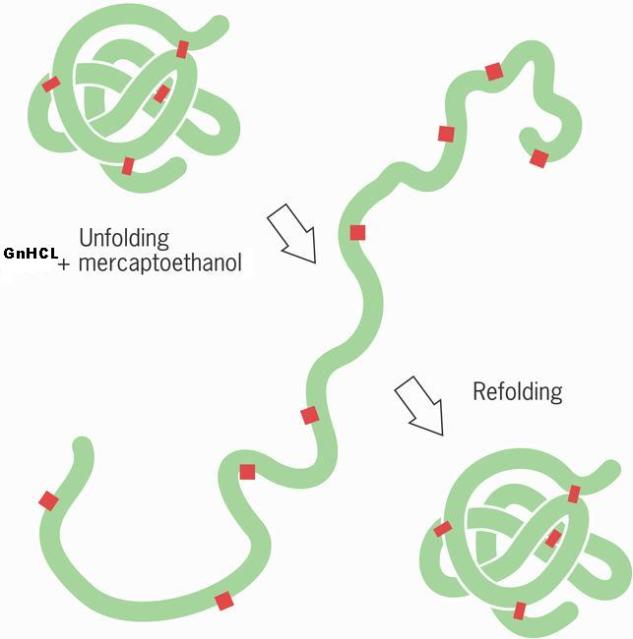
They can be denatured by heat, chemicals, and even changes in pH, this was shown in Anfinsen’s experiment. Here he demonstrated that the protein ribonuclease when denatured it unfolded under the conditions he introduced, but when he removed the conditions the protein was able to reform its original structure. The chemicals Anfinsen used were GnHCL which is a chaotrope and mercaploethanol which acted as a reducing agent by making the covalent bonds break. The covalent bonds were di sulfide bonds, to remove the chemicals he used dialysis tubing that had small holes, the holes allowed the chemicals to pass but prevented the unfolded protein to pass because it was too big. Note that the primary structure of proteins are not broken down when proteins are denatured.
Proteins can be either globular (water soluble) or fibrous (not water soluble). Examples of globular proteins are those found in the egg white and an example of a fibrous protein is keratin which is found in hair and nails.
In this class what was thought about amino acids was applied to the structure of proteins hence the biochemistry of proteins are solely due to that of their amino acid residues.
With this knowledge of the sequence of proteins it is possible to detect how mutations of DNA affect the protein structure as seen with hemoglobin in red blood cells. Here we know the structure of normal hemoglobin and it was possible to detect the difference in the amino acid sequence in hemoglobin found in a sickle red blood cell. So we can apply this knowledge in sequencing other proteins and this can help us to find the causes of diseases and see how the mutation of DNA can result in drastic changes in the structure of proteins. We know how protein structure is affected and we can determine how well a protein can function under certain conditions. We can optimize the application of proteins in everyday life such as with enzymes and the manufacture of products such as yogurt.
There is much more information on proteins that can be found using my references:
Wikipedia the free encyclopedia. “Protein”. Accessed 10th march 2013. http://en.wikipedia.org/wiki/Protein
DONALD VOET, JUDITH G. VOET.2004. Biochemistry, 4th edition. Unites states of America: JOHN WILEY & SONS , INC.